Palladium-catalyzed β-selective C(sp2)–H carboxamidation of enamides by isocyanide insertion: synthesis of N-acyl enamine amides†
Abstract
An efficient synthesis of N-acyl enamine amides via palladium-catalyzed alkene C–H activation and isocyanide insertion has been developed. The reaction was realized with good regioselectivity through directing-group-assisted alkenyl C(sp2)–H bond carboxamidation under mild conditions. A facile pathway to β-amino amides was achieved via reduction of the synthesized N-acyl enamine amides.
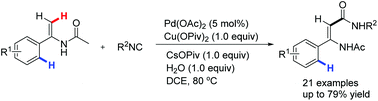

 Please wait while we load your content...
Please wait while we load your content...