(1S)-(−)-N-Trifluoromethylthio-2,10-camphorsultam and its derivatives: easily available, optically pure reagents for asymmetric trifluoromethylthiolation†
Abstract
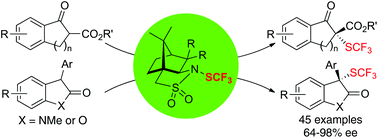
A family of easily scalable, shelf-stable, optically pure reagents (1S)-(−)-N-trifluoromethylthio-2,10-camphorsultam 1a–c was successfully developed. In particular, compound 1c was shown to be an efficient reagent that is capable of transferring chirality to other prochiral nucleophiles such as β-ketoesters, oxindoles and benzofuranones with good to excellent enantioselectivities.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017

 Please wait while we load your content...
Please wait while we load your content...