Functionalization of pentacene-5,7,12,14-tetraone with geminal enediyne and 1,3-dithiole groups†
Abstract
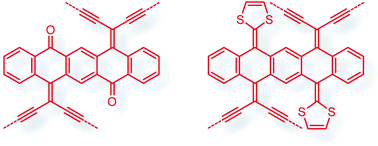
Pentacene-5,7,12,14-tetraone was subjected to selective olefination and cross-coupling reactions to yield a new class of pentacene-based π-conjugated systems functionalized with geminal enediyne and 1,3-dithiole groups. The electron donating and accepting effects of enediyne and dithiole groups render these compounds intriguing structural, electronic properties, and redox activities which were systematically investigated by UV-Vis absorption and cyclic voltammetric analyses in conjunction with density functional theory (DFT) calculations. The bis(enediyne)-substituted pentacenedione showed potential application in the preparation of carbon-rich polymeric materials.
- This article is part of the themed collection: Novel π-electron molecular scaffolds


 Please wait while we load your content...
Please wait while we load your content...