Synthesis of β-keto sulfones via a multicomponent reaction through sulfonylation and decarboxylation†
Abstract
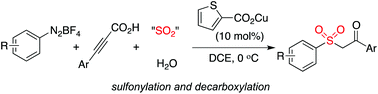
A copper(I)-catalyzed synthesis of β-keto sulfones through a multicomponent reaction of aryldiazonium tetrafluoroborates, 3-arylpropiolic acids, sulfur dioxide, and water was developed. This reaction proceeds through a tandem radical process, and the sulfonyl radical, generated from the combination of aryldiazonium tetrafluoroborates with DABCO·(SO2)2, acts as the key intermediate. The transformation involves sulfonylation and decarboxylation, which allows for the efficient synthesis of the desired β-keto sulfones.

 Please wait while we load your content...
Please wait while we load your content...