I2-Catalyzed sulfenylation of indoles and pyrroles using triethylammonium thiolates as sulfenylating agents†
Abstract
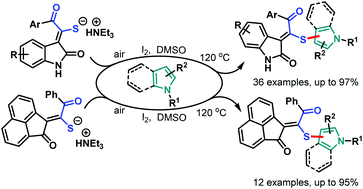
Readily available triethylammonium thiolates were proved to be new and eco-friendly sulfenylating agents for the efficient and practical construction of sulfenylated indoles and pyrroles (48 examples) with good to excellent yields under metal-free and microwave irradiation conditions. The combination of I2 and DMSO enabled direct C–S bond formation, allowing easy and low-cost access to new functionalized C,S-tethered bisindoles and pyrrole–indole pairs with a wide diversity of substituents. The mechanism involving S–S and S–I bond-forming/breaking events was proposed.


 Please wait while we load your content...
Please wait while we load your content...