Decarboxylative Umpolung of conjugated enals to β-carbanions for intramolecular nucleophilic addition to an aldehyde†
Abstract
An interesting Umpolung strategy to generate β-carbanion equivalents from a conjugated enal has been developed. The enal group of compounds 7 was chemospecifically converted to a delocalized carbanion by decarboxylation of the in situ formed imine between the enal and 2,2-diphenylglycine (2), which then underwent nucleophilic addition at the β position of the enal to an intramolecular aldehyde group to give cyclized products 8 in 54–93% yields.
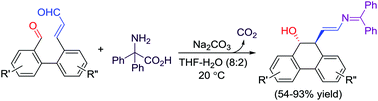


 Please wait while we load your content...
Please wait while we load your content...