KBr/K2S2O8-mediated dibromohydration of N-(2-alkynylaryl)acetamide†
Abstract
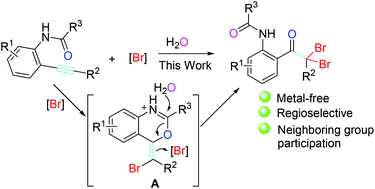
The regioselective dibromohydration of N-(2-alkynylaryl)acetamide with the participation of a neighbouring acetamino group is described here for the synthesis of N-(2-(2,2-dibromo-2-arylacetyl)aryl)acetamide. The dibromohydration proceeds under mild conditions and works well without metal catalysis. More importantly, by employing different neighbouring groups, switchable synthesis can be achieved. The entire process of the dibromohydration of N-(2-alkynylaryl)acetamide consists of KBr/K2S2O8-based electrophilic 6-exo cyclization to form bromo-containing benzoxazine cation and the oxidative ring-opening of the resulting bromo-containing benzoxazine cation. Water serves as a reagent to be incorporated into the acetamino group.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...