Iodine(iii)-induced regioselective carbocyclization of terminal alkynes: a facile approach to prepare 1,1-diiodomethylene substituted cyclic compounds†
Abstract
An iodine(III)-induced regioselective intramolecular carbocyclization of functionally substituted terminal alkynes is reported. A range of 1,1-diiodomethylene substituted cyclic compounds were prepared by using iodide salts as the iodine source to replace the normally used electrophilic I2, NIS or ICl. The use of 2,2,2-trifluoroethanol as a solvent is essential for the generation of reactive iodine(III) species. The resulting diiodomethylene substituted cyclic compounds can be readily converted to tetra-substituted alkenes through transition metal-catalyzed coupling reactions.
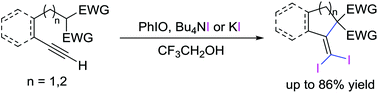


 Please wait while we load your content...
Please wait while we load your content...