Efficient synthesis of multiply substituted butenolides from keto acids and terminal alkynes promoted by combined acids†
Abstract
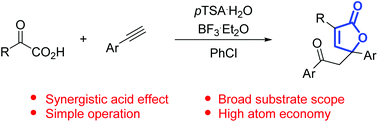
A general, efficient, and operationally simple approach to highly substituted butenolides through the annulation of keto acids and terminal alkynes is described. This reaction is promoted by the combination of Lewis and Brønsted acids, furnishing a variety of butenolides in synthetically useful yields.


 Please wait while we load your content...
Please wait while we load your content...