De novo synthesis of alkyne substituted tryptophans as chemical probes for protein profiling studies†
Abstract
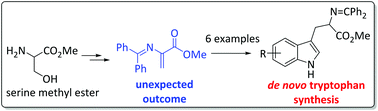
De novo synthesis of alkynylated tryptophan moieties as chemical probes for protein profiling studies, via an unexpected synthesis of Michael acceptor 12 is reported. Friedel Craft's alkylation of 12 and alkyne substituted indoles gave alkynylated tryptophan moieties, which act as chemical probes by incorporating into actively translating Escherichia coli cells, whereby the alkyne moiety enables multimodal analyses through click chemistry mediated attachment of reporting groups.


 Please wait while we load your content...
Please wait while we load your content...