Synthesis of 3-acylquinolines through Cu-catalyzed double C(sp3)–H bond functionalization of saturated ketones†
Abstract
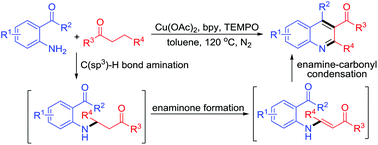
A novel synthesis of 3-acylquinolines from Cu-catalyzed one-pot reactions of 2-aminoaryl aldehydes/ketones with inactivated ketones is presented. Mechanistically, the formation of the title compounds involves a cascade procedure including C(sp3)–H bond amination, enaminone formation, and enamine-carbonyl condensation. To our knowledge, this should be the first example in which 3-acylquinolines are prepared through Cu-catalyzed double C(sp3)–H bond functionalization of saturated ketones.


 Please wait while we load your content...
Please wait while we load your content...