Silver-catalyzed geminal aminofluorination of diazoketones with anilines and N-fluorobenzenesulphonimide†
Abstract
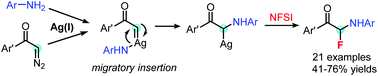
A silver-catalyzed three-component reaction of diazoketones, anilines and NFSI is developed. The reaction provides a new method for gem-aminofluorination of acceptor diazo compounds with aniline as the nitrogen source and NFSI as the fluorine source.


 Please wait while we load your content...
Please wait while we load your content...