Biomimetic total synthesis of (±)-berkeleyamide D†
Abstract
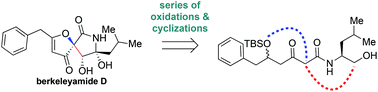
We describe the total synthesis of (±)-berkeleyamide D using a biosynthetically inspired strategy. The spirocyclic core of berkeleyamide D was constructed via sequential epoxidations and a late-stage base-mediated cyclization of a biosynthetically relevant precursor. Our synthetic solution might be applicable to access other fungal natural products of this family.


 Please wait while we load your content...
Please wait while we load your content...