Asymmetric hydrogenation of α-hydroxy ketones with an iridium/f-amphox catalyst: efficient access to chiral 1,2-diols†
Abstract
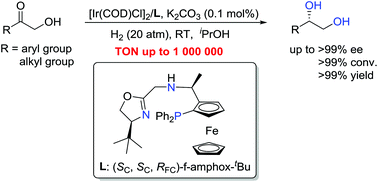
We successfully applied tridentate f-amphox ligands to the iridium-catalytic asymmetric hydrogenation of various α-hydroxy ketones to afford the corresponding chiral 1,2-diols with excellent results (almost all products up to 99% yield and >99% ee). The resulting chiral 1,2-diols and their derivatives are important synthetic and pharmaceutical intermediates. Our catalytic system displayed extremely high activity, achieving up to 1 000 000 turnover number (TON). The great performance of this asymmetric hydrogenation makes the preparation of various chiral 1,2-diols highly practical with great potential.


 Please wait while we load your content...
Please wait while we load your content...