Synthesis of tetrasubstituted symmetrical pyridines by iron-catalyzed cyclization of ketoxime acetates†
Abstract
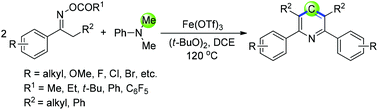
An efficient iron-catalyzed cyclization of ketoxime acetates with N,N-dimethylaniline for the synthesis of symmetrical pyridines has been developed. A methyl carbon on N,N-dimethylaniline acts as a source of one carbon synthons. The reaction used readily available starting materials, tolerated various functional groups, and afforded 2,3,5,6-tetrasubstituted symmetrical pyridines in good yields.


 Please wait while we load your content...
Please wait while we load your content...