The synthesis of unsymmetric diamides through Rh-catalyzed selective C–H bond activation of amides with isocyanates†
Abstract
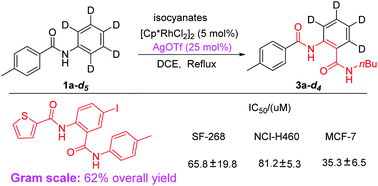
Rh-catalyzed selective C–H bond activation was achieved with the assistance of non-coordinating anions. This methodology has been shown to excel as a means to selectively activate N-aryl rings for the synthesis of unsymmetric diamides, while the reaction could not occur without non-coordinating anions, which provides effective proof that non-coordinating anions play an important role in C–H bond activation. The results of mechanism experiments definitely eliminated the possibility of the “silver effect” in this silver-involving transformation. Importantly, this provided an efficient method for the synthesis of N-(4-iodo-2-(p-tolylcarbamoyl)phenyl)thiophene-2-carboxamide in an overall yield of 62% using just three steps, which exhibited promising activity against cancer-cell lines.

 Please wait while we load your content...
Please wait while we load your content...