Phosphine-mediated domino reactions of phthalimidomalonates with allenoates or but-2-ynoate: facile entry into highly functionalized pyrroloisoindolinone derivatives†
Abstract
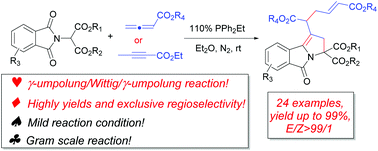
A phosphine-mediated domino reaction between phthalimidomalonates and allenoates furnishes highly functionalized pyrroloisoindolinone derivatives in synthetically useful yields. When ethyl but-2-ynoate was reacted with phthalimidomalonates under the same conditions, pyrroloisoindolinone derivatives were also obtained in good to excellent yields. The mechanism for the transformation is a tandem γ-umpolung/Wittig/γ-umpolung process. The present domino reaction not only exploits the potential of allenoates, but also extends the existing phosphine-mediated cyclization scope.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017

 Please wait while we load your content...
Please wait while we load your content...