A copper-catalyzed arylation/nucleophilic addition/fragmentation/C–S bond formation cascade: synthesis of bis(arylthio)imines†
Abstract
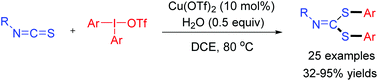
A new tandem arylation/nucleophilic addition/fragmentation/C–S bond formation reaction involving diaryliodonium salts as electrophiles was developed with Cu(OTf)2 as the catalyst. A series of unexpected bis(arylthio)imine derivatives were generated in good yields. The reaction proceeds under mild conditions, and the bis(arylthio)imines are readily transformed to other useful products.


 Please wait while we load your content...
Please wait while we load your content...