Total synthesis of I3,II8-biapigenin and ridiculuflavone A†
Abstract
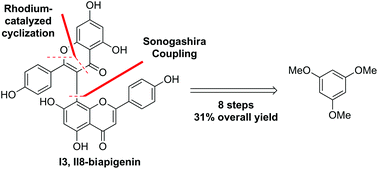
The first total synthesis of I3,II8-biapigenin and ridiculuflavone A via the key steps of regioselective iodination, Sonogashira reaction, and rhodium-catalyzed oxidative coupling with the overall yields of 31% and 22%, respectively, was reported. The structures of the key intermediates for the two natural products were confirmed by single-crystal X-ray analysis.


 Please wait while we load your content...
Please wait while we load your content...