Sequential In-catalyzed intramolecular hydroarylation and Pd-catalyzed cross-coupling reactions using bromopropargyl aryl ethers and amines†
Abstract
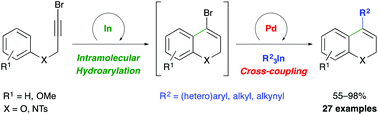
A sequential one-pot indium-catalyzed intramolecular hydroarylation (IMHA) of bromopropargyl aryl ethers and amines, and palladium-catalyzed cross-coupling reaction using triorganoindium reagents (R3In) has been developed. In this transformation, the IMHA of 3-bromo-2-propynyl aryl ethers under indium(III) catalysis, proceeds regioselectively through a 6-endo dig pathway to afford 4-bromo-2H-chromenes. Subsequent palladium-catalyzed cross-coupling with R3In gives 4-substituted-2H-chromenes in one-pot. This sequential transformation was extended to 3-bromo-2-propynyl-N-tosylanilines to afford 4-substituted-1,2-dihydroquinolines. The dual-catalyzed procedure takes place efficiently with a variety of propargyl aryl ethers and amines and R3In (R = aryl, heteroaryl, alkyl or alkynyl), showing the efficiency of these organometallics and proving the compatibility of indium and palladium in catalysis.


 Please wait while we load your content...
Please wait while we load your content...