Substrate-driven selective mono- and bis-couplings of ortho-(OTf/I/Br) substituted gem-dibromovinylarenes†
Abstract
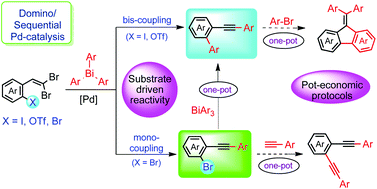
A one-pot approach with the substrate-driven selective and competitive reactivity of ortho-trifloxy, iodo or bromo functionalized gem-dibromovinylarenes was studied under Pd-catalyzed conditions. These investigations provided synthetically important and structurally diverse functional ortho-alkynylbiaryls, ortho-alkynylbromoarenes, arene-1,2-diynes and diarylmethylidenefluorenes. The adopted protocol involving the in situ generation of 1-bromoalkyne from gem-dibromovinylarenes and their functionalization in pot- and atom-economical couplings employing triarylbismuths furnished a viable synthetic methodology with good to high yields. The present study also unveiled the substrate-driven selective mono- and bis-couplings using palladium catalyzed domino and sequential reaction protocols.

 Please wait while we load your content...
Please wait while we load your content...