Iridium catalysts with modular axial-unfixed biphenyl phosphine–oxazoline ligands: asymmetric hydrogenation of α,β-unsaturated carboxylic acids†
Abstract
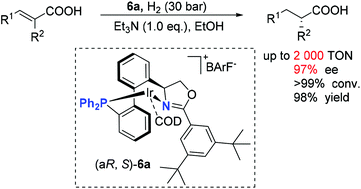
A series of highly modular chiral axial-unfixed biphenyl phosphine–oxazoline ligands were developed via a simple synthetic route using readily available (S)-(+)-2-phenylglycinol as a starting material. Modular oxazoline motifs can be efficiently constructed through various easily accessible carboxylic acids. These ligands were successfully applied to Ir-catalyzed asymmetric hydrogenation of α,β-unsaturated carboxylic acids to afford chiral α-substituted carboxylic acids with excellent results (up to 97% ee, 98% yield, 2000 TON).
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...