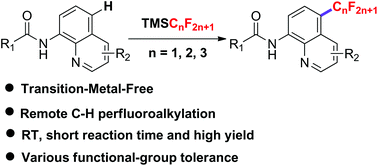
Transition-metal-free direct perfluoroalkylation of quinoline amides at C5 position through radical cross-coupling under mild conditions†
Abstract
A simple and efficient method is established for the synthesis of perfluoroalkylated quinolines in the absence of any transition metal catalysts. The eco-friendly reaction reveals lower energy and time consumption. Desired products are isolated in good to excellent yields. A number of control experiments are carried out to illustrate that a radical cross-coupling process plays a vital role in the perfluoroalkylation.

 Please wait while we load your content...
Please wait while we load your content...