Copper(i) and N-fluorobenzenesulfonimide-mediated direct regioselective halogenation of 8-amidoquinolines on the C5 position†
Abstract
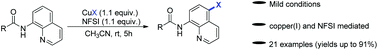
An efficient and convenient method for the direct regioselective halogenation of 8-amidoquinolines has been developed. In the presence of CuX and N-fluorobenzenesulfonimide (NFSI), the C5-halogenation products of 8-acylaminoquinolines were obtained in moderate to excellent yields. This new procedure provides facile access to a variety of C5-halogenation of 8-amidoquinolines under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...