Catalytic enantioselective bromohydroxylation of aryl olefins with flexible functionalities†
Abstract
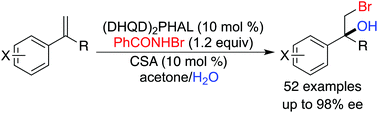
A highly enantioselective bromohydroxylation of aryl olefins with flexible functionalities has been achieved with (DHQD)2PHAL as a catalyst and H2O as a nucleophile, giving a variety of optically active bromohydrins with up to 98% ee. An acid additive has been found to be beneficial for the reactivity and enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...