Natural diarylfluorene derivatives: isolation, total synthesis, and phosphodiesterase-4 inhibition†
Abstract
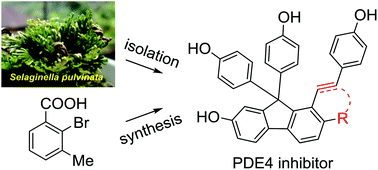
In our previous study, selaginpulvilins A–D (1–4) featuring an unprecedented 9,9-diphenyl-1-(phenylethynyl)-9H-fluorene skeleton were identified as potent phosphodiesterase-4 (PDE4) inhibitors from Selaginella pulvinata. In the current work, a large-scale reinvestigation of the same plant led to the isolation of six additional new analogues, selaginpulvilins E–J (5–10), among which 5 features a rare 6-(4-hydroxyphenyl)-2H-pyran-2-one unit. Compounds 5–10 exhibited remarkable inhibitory activities against PDE4 with IC50 values in the range of 0.22–1.38 μM. The first total synthesis of selaginpulvilins A–F (1–6) was developed in 7–11 steps involving a Friedel–Crafts reaction as the key reaction, which provides a feasible access to this scaffold.

 Please wait while we load your content...
Please wait while we load your content...