Transition-metal free direct difluoromethylthiolation of electron-rich aromatics with difluoromethanesulfonyl chloride†
Abstract
A new method for phosphine-mediated difluoromethylthiolation of indole derivatives and other electron-rich aromatics including pyrroles, pyrazolones, indolizine, and 1,3,5-trimethoxybenzene was developed using difluoromethanesulfonyl chloride. The additive n-Bu4NI was demonstrated to facilitate this reaction by generating iodine in situ, which could promote this transformation. The use of a transition-metal-free protocol, readily available reagents, and mild reaction conditions makes this protocol more practical than traditional methods.
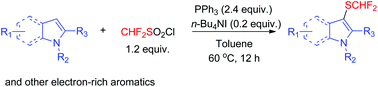

 Please wait while we load your content...
Please wait while we load your content...