Copper-mediated annulation of 2-(1-arylvinyl) anilines and aryl nitrosos towards 2,3-diaryl-2H-indazoles†
Abstract
A copper-mediated annulation of 2-(1-substituted vinyl) anilines and aryl nitrosos is developed for the synthesis of 2,3-diaryl pyrazoles. Compared with previously reported sequential azobenzene C–H ortho-acylation, reduction and cyclization procedures towards such frameworks, no external reductant was required during this cyclization, where the vinyl served as a formal innate reductant. Moreover, in this procedure, no selectivity was involved in the case of Ar1 ≠ Ar2.
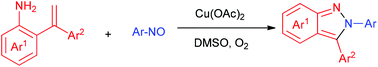
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2016

 Please wait while we load your content...
Please wait while we load your content...