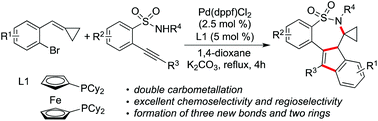
Synthesis of polycyclic sultams via a palladium-catalyzed reaction of 1-bromo-2-(cyclopropylidenemethyl)benzenes with 2-alkynylbenzenesulfonamides†
Abstract
Polycyclic sultams are efficiently synthesized through a palladium-catalyzed tandem reaction of 1-bromo-2-(cyclopropylidenemethyl)benzenes with 2-alkynylbenzenesulfonamides. The transformation proceeds through double carbometallation with excellent chemoselectivity and regioselectivity, leading to a range of 7,7a-dihydro-6H-benzo[f]indeno[1,2-d][1,2]thiazepine 5,5-dioxides in moderate to good yields. During the reaction process, three new bonds and two rings including the seven-membered sultam ring are formed.

 Please wait while we load your content...
Please wait while we load your content...