Pyridine coupled mono and bisbenzimidazoles as supramolecular gelators: selective metal ion sensing and ionic conductivity†
Abstract
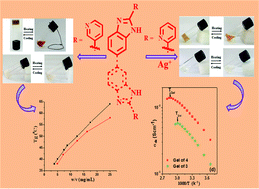
Pyridine coupled mono and bisbenzimidazoles 1–6 are synthesized and their gelation properties are examined in various polar as well as nonpolar solvents. Among the structures, compounds 1 and 2 form instant gels from DMSO/H2O and MeOH/H2O solvents. The gel states of both 1 and 2 are responsive to Ag+ and Cu2+ ions whereas the gel to sol transition of 2 in DMSO–H2O is additionally caused by Hg2+ ions. On the other hand, compounds 3 and 4 produce gels only in the presence of Ag+ ions under similar conditions and validate their visual readout. In relation to this, the nongelation behavior of p-isomers 5 and 6 (structural isomers) under identical conditions emphasizes the positional role of the pyridyl ring nitrogen with respect to the imidazole ring. Furthermore, metallogels of 3 and 4 exhibit thermally activated ionic conductivity due to movement of Ag+ ions within the gel network.


 Please wait while we load your content...
Please wait while we load your content...