Hidden complexity of synergistic roles of Dopa and lysine for strong wet adhesion†
Abstract
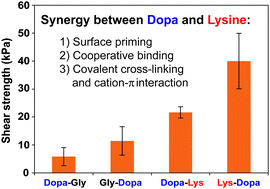
Dopa and lysine are widely found in mussel foot proteins and are suggested to play synergistic roles in wet adhesion; yet, the detailed molecular mechanism remains unclear. Here, using PEG conjugated dipeptides as the model system, we found that the neighboring lysine can significantly enhance surface binding of Dopa through three distinct mechanisms: (1) displacing surface water and ions to increase the effective binding sites; (2) being directly involved in cooperative surface binding in a sequence dependent manner; (3) enhancing cohesion by Michael addition to oxidized species or forming cation–π interactions. This study may be helpful for rational design of biomimetic strong adhesives for biomedical applications.
- This article is part of the themed collection: 2017 Emerging Investigators by MCF


 Please wait while we load your content...
Please wait while we load your content...