Synthesis and self-assembly of unconventional C3-symmetrical trisubstituted triphenylenes†
Abstract
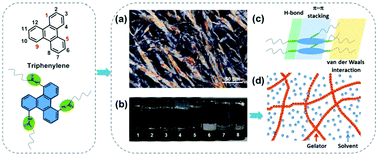
Triphenylene-based self-assembly modules, bearing three amide groups at the 1-, 5-, and 9-positions, were designed and synthesized. Supramolecular gels could be constructed by combining hydrogen bonding, π–π stacking and van der Waals interactions. In addition, the assembly exhibited liquid crystalline mesophases by tuning of the length of the alkyl chains. This work provides an essential representative of functional assembly based on C3-symmetric triphenylenes.
- This article is part of the themed collections: 2017 Emerging Investigators by MCF and Materials Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...