Multicarbazolyl substituted TTM radicals: red-shift of fluorescence emission with enhanced luminescence efficiency†
Abstract
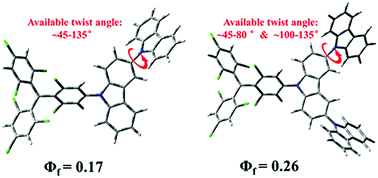
A series of multicarbazolyl substituted tris(2,4,6-trichlorophenyl)-methyl (TTM) radical derivatives were synthesized. Red-shifts of the fluorescence emission of the TTM radicals were achieved along with enhanced luminescence efficiency through incorporating substituent groups with strong electron donating ability as well as restricting the rotation of the outer groups. The photostability of the radicals was also significantly enhanced via the incorporation of substituent groups. This work provides a new approach to realize NIR-emitting radicals with high luminescence efficiency.
- This article is part of the themed collection: Molecular Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...