A glutamic acid-modified cellulose fibrous composite used for the adsorption of heavy metal ions from single and binary solutions†
Abstract
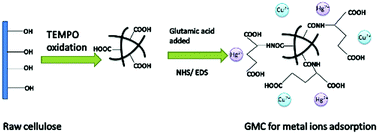
A new glutamic acid-modified cellulose was prepared and investigated as a bioadsorbent used for the simple and rapid removal of Cu2+ and Hg2+ from single and binary aqueous solutions. The effects of the initial concentration of heavy metal ions, pH of the solution and temperature on the adsorption capacity of the bioadsorbent were investigated. Equilibrium studies demonstrate that the adsorption of Cu2+ and Hg2+ follows the Langmuir model in a single aqueous solution system. Meanwhile, the adsorption kinetics were found to follow the pseudo-second-order model well. Competitive adsorption in the binary component system indicates the mutual inhibitation adsorption between the two metal ions. The FTIR and SEM–EDS results reveal that Cu2+ and Hg2+ are successfully adsorbed on the bioadsorbent by coordination and static interactions.
- This article is part of the themed collection: Molecular Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...