Direct amination of the antiaromatic NiII norcorrole†
Abstract
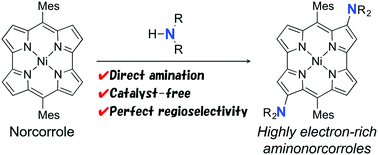
We have discovered a facile amination reaction of NiII dimesitylnorcorrole, which is an antiaromatic porphyrinoid with a 16π-electron conjugation system. The amination reaction proceeded at the β-positions of the norcorrole with high regioselectivity upon treatment with various amines used as solvents. The reaction requires neither catalysts nor pre-functionalization of the norcorrole. The optical and electrochemical properties of the amino norcorroles were substantially modulated by electron-donating nitrogen atoms. According to theoretical studies, the regioselectivity of amination can be accounted for by molecular orbitals of the norcorrole.
- This article is part of the themed collections: Pi conjugated system bricolage (figuration) toward functional organic molecular systems, Materials Chemistry Frontiers HOT articles for 2017 and MCF Editors’ Recommendation


 Please wait while we load your content...
Please wait while we load your content...