A photoacid generator integrated terpolymer for electron beam lithography applications: sensitive resist with pattern transfer potential†
Abstract
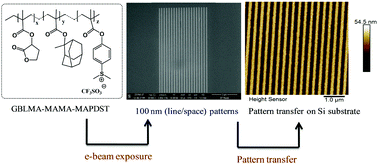
While developing a new resist for different lithography applications, starting from high to low nodes, the potential of the resist in successful pattern transfer has been the key for practical applications particularly in semiconductor industries. Although semiconductor industries are looking for materials for sub 7 nm node technology for sophisticated electronic appliances, the materials with potential for high resolution larger node pattern transfer are equally important for specialized applications in CMOS technology. In this regard, a new ionic photoacid generator included terpolymer photoresist viz GBLMA–MAMA–MAPDST has been synthesized for next generation lithography (NGL) applications. Electron beam lithography (EBL) studies of this resist coated thin films have shown that the resist can pattern 100 nm line/space features under e-beam exposure. The sensitivity (E0) and contrast (γ) were calculated to be 36.5 μC cm−2 and 0.08 respectively. Finally, transfer of 100 nm (line/space) patterns into a silicon substrate has been achieved using a dry plasma etching technique.


 Please wait while we load your content...
Please wait while we load your content...