Multi-stimuli responsive luminescent azepane-substituted β-diketones and difluoroboron complexes†
Abstract
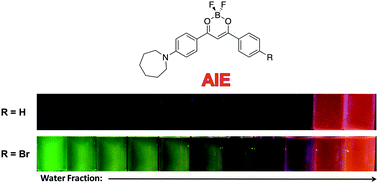
Difluoroboron β-diketonate (BF2bdk) compounds show environment-sensitive optical properties in solution, aggregation-induced emission (AIE) and multi-stimuli responsive fluorescence switching in the solid state. Here, a series of 4-azepane-substituted β-diketone (bdk) ligands (L-H, L-OMe, L-Br) and their corresponding difluoroboron dyes (D-H, D-OMe, D-Br) were synthesized, and various responsive fluorescence properties of the compounds were studied, including solvatochromism, viscochromism, AIE, mechanochromic luminescence (ML) and halochromism. Compared to the β-diketones, the boron complexes exhibited higher extinction coefficients but lower quantum yields, and red-shifted absorption and emission in CH2Cl2. Computational studies showed that intramolecular charge transfer (ICT) dominated rather than π–π* transitions in all the compounds regardless of boron coordination. In solution, all the bdk ligands and boron dyes showed red-shifted emission in more polar solvents and increased fluorescence intensity in more viscous media. Upon aggregation, the emission of the β-diketones was quenched, however, the boronated dyes showed increased emission, indicative of AIE. Solid-state emission properties, ML and halochromism, were investigated on spin cast films. For ML, smearing caused a bathochromic emission shift for L-Br, and powder X-ray diffraction (XRD) patterns showed that the “as spun” and thermally annealed states were more crystalline and the smeared state was amorphous. No obvious ML emission shift was observed for L-H or L-OMe, and the boronated dyes were not mechano-active. Trifluoroacetic acid (TFA) and triethylamine (TEA) vapors were used to study halochromism. Large hypsochromic emission shifts were observed for all the compounds after exposure to TFA vapor, and reversible fluorescence switching was achieved using the acid/base pair.
- This article is part of the themed collection: Aggregation-Induced Emission


 Please wait while we load your content...
Please wait while we load your content...