Flower-shaped cobalt oxide nano-structures as an efficient, flexible and stable electrocatalyst for the oxygen evolution reaction†
Abstract
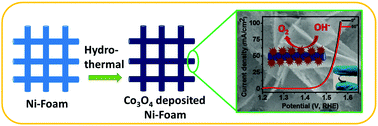
The industrial application of water splitting for oxygen evolution requires low cost, high performance and stable electrocatalysts which can operate at low overpotential. Here, we develop a high performance and stable electrocatalyst for the oxygen evolution reaction (OER) using earth abundant materials. A binder free approach for the synthesis of flower-shaped cobalt oxide (Co3O4) composed of nanosheets showed high OER catalytic activity. The Co3O4 electrode requires a low overpotential of 356 mV to achieve a current density of 10 mA cm−2 with a low onset potential of 284 mV. The electrode showed outstanding flexibility and stability. The catalytic activity of the Co3O4 electrode was very stable up to the 2000th cycle of the polarization study. The high catalytic activity and structural stability arise due to efficient and fast charge transportation through the nanosheets of Co3O4 which are in direct contact with the conducting nickel of the electrode. The porous structure of Co3O4 allows easy access of the electrolyte and escape of generated oxygen without damaging the structure. Collectively, the flower-shaped nanostructured Co3O4 electrode can be used as a flexible and high performance electrode for the OER in an industrial setup.


 Please wait while we load your content...
Please wait while we load your content...