Non-conjugated fluorescent molecular cages of salicylaldehyde-based tri-Schiff bases: AIE, enantiomers, mechanochromism, anion hosts/probes, and cell imaging properties†
Abstract
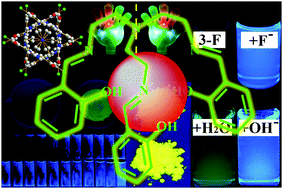
Luminescent organic materials are commonly polycyclic aromatic molecules with planar extended π-electron conjugation. In the present work, however, we report a series of novel and simple salicylaldehyde-based tri-Schiff bases (TSBs, 15 samples), which have a non-conjugated trimethylamine bridge but emit strong blue, green, and yellow aggregation-induced emission (AIE) with large Stokes shifts (up to 167 nm) and high fluorescence quantum yields (up to 0.18). Mechanochromic fluorescence enhancement and enantiomers are also found in TSB solids. Moreover, these flexible and tripod-like molecular cages acting as ideal and universal anion hosts can be used to detect anions with turn-on fluorescence signals (fluorescence quantum yields up to 0.51). Combining their advantages of AIE and biocompatibility, TSBs have potential application in living cell imaging. The inherent relationships between their chemical structures and AIE/anion host properties are studied, which provide unequivocal insights for the design of non-conjugated fluorescent materials.


 Please wait while we load your content...
Please wait while we load your content...