Insights into the origin of aggregation enhanced emission of 9,10-distyrylanthracene derivatives†
Abstract
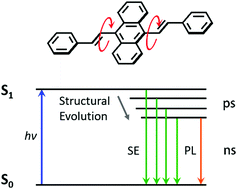
9,10-Distyrylanthracene (DSA) and its four derivatives are investigated by both steady state and ultrafast spectroscopy to reveal the intrinsic photophysical process upon excitation. Intramolecular rotation around the vinyl moiety plays an important role in the whole photophysical process in addition to the electronic properties of the peripheral substituents. In dilute solutions, DSA derivatives possess a twisted structure in the ground state that eventually relaxes to a planar structure within picoseconds. The fluorescence process is dominated by the relaxed excited state, and the quantum yield is affected by competition between the nonradiative and radiative deactivations. The enhanced fluorescence of the molecular aggregates originates from the optically allowed S1–S0 transition together with the suppressed nonradiative deactivation via molecular stacking. These findings provide an in-depth understanding of the origin of the aggregation enhanced emission process, and may be applicable for the fine design of DSA based molecules with enhanced fluorescence and novel structures beyond DSA.
- This article is part of the themed collections: Celebrating Excellence in Research: Women at the Frontiers of Chemistry, Materials Chemistry Frontiers HOT articles for 2017 and Aggregation-Induced Emission


 Please wait while we load your content...
Please wait while we load your content...