A facile method for the synthesis of a C@MoO2 hollow yolk–shell structure and its electrochemical properties as a faradaic electrode†
Abstract
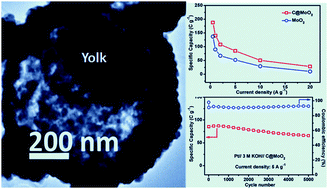
Transition-metal oxide hollow yolk–shell micro/nanostructures combined with a conducting substance have gained significant attention as efficient electrode materials for electrochemical energy storage applications due to their large surface area, internal void space, and structural stability. Herein, we report a facile aqueous solution-based soft template method using sucrose–CTAB for the synthesis of a hollow yolk–shell structure of carbon-incorporated MoO2 (C@MoO2) with a diameter of 0.9–1.1 μm, wall thickness of 100 nm, inner yolk size of 400–450 nm, and BET surface area of 40 m2 g−1. During the synthesis process, sucrose plays a dual role, both as a template and a carbon source. The electrochemical charge storage mechanism follows a battery-type behaviour when tested as a faradaic electrode in 3.0 M KOH electrolyte. C@MoO2 exhibits a high specific capacity of 188 C g−1 at the current density of 0.5 A g−1, good rate performance (50.6 C g−1 at 10 A g−1), and 78% retention of capacity after 5000 cycles at 5 A g−1. The obtained performance is superior to those obtained for pure MoO2 hollow spheres (137.1 C g−1 at 0.5 A g−1) as well as previously reported MoO2 and MoO3, indicating the potential applicability of the as-synthesised yolk–shell C@MoO2.


 Please wait while we load your content...
Please wait while we load your content...