Benzothiazoles-substituted tetraphenylethylenes: synthesis, structure, aggregation-induced emission and biological studies†
Abstract
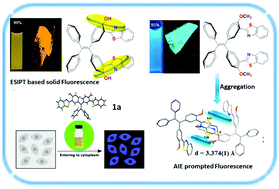
New tetraphenylethylene (TPE) bearing benzothiazoles at the ortho-position to OH and OCH3 groups, 1a and 1b, respectively, were prepared. Benzothiazole tetraphenylethylenes 1a/1b were synthesized by the acid-catalysed condensation reaction of an appropriate di-aldehyde, such as 5,5′-(2,2-diphenylethylene-1,1-diyl)bis(2-hydroxybenzaldehyde) 2a or 5,5′-(2,2-diphenylethylene-1,1-diyl)bis(2-methoxybenzaldehyde) 2b, with 2-aminothiophenol. Compound 1a exhibited an excited state intramolecular proton transfer (ESIPT) phenomenon. The photophysical properties of compounds 1a/1b in solution and aggregated form, along with their structure–property relationships, were comparatively investigated. The study showed that the substituent groups on TPE at the ortho position to benzothiazole have a great influence on the electronic structure, molecular packing, and aggregation-induced emission properties. Moreover, the cell viability studies by MTT assay and fluorescence cell imaging experiments proved the low-toxicity of 1a and that it can be used as a potential contrasting agent in cell imaging studies.


 Please wait while we load your content...
Please wait while we load your content...