Green light-emitting 2-(1H-indol-3-yl)acetonitrile-based D–A fluorophores – a combined theoretical and experimental study†
Abstract
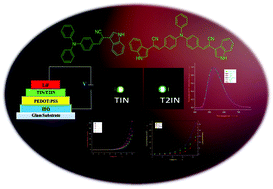
A series of new fluorophores based on 2-(1H-indol-3-yl)acetonitrile incorporated with various polycyclic aromatic hydrocarbons, carbazole, pyridine, and triphenylamine was designed and synthesised. The optical, electrochemical, thermal, and morphological properties of the fluorophores were studied using various spectroscopic techniques. The demonstration of electroluminescence behaviour was carried out by fabricating a single layer device with the device structure of indium tin oxide (ITO – 150 nm)/poly(ethylenedioxythiophene)–polystyrene sulfonate (PEDOT:PSS – 40 nm)/emissive layer (65 nm)/lithium fluoride (LiF – 0.5 nm)/Al (120 nm), which exhibited a turn-on voltage of 6.5 V, maximum brightness of 110 cd m−2 and 133 cd m−2, maximum current efficiency of 0.34 cd A−1 and 0.73 cd A−1, and power efficiency of 0.10 lm W−1 and 0.28 lm W−1 for TIN and T2IN, respectively. Density functional theory (DFT) results revealed that the highest occupied molecular orbital (HOMO) is mainly localized on the donor part, whereas the lowest unoccupied molecular orbital (LUMO) is spread over the entire molecule i.e. over the donor, π–spacer, and acceptor units. The higher HOMO and lower LUMO energies of fluorophores reduce the energy barrier for hole creation and electron acceptance processes, respectively. All the fluorophores show a narrow band gap, and the charge injection ability increases with suitable substitution, which leads to improved organic light-emitting diode (OLED) performance.

 Please wait while we load your content...
Please wait while we load your content...