Increasing the stability of Mg2(dobpdc) metal–organic framework in air through solvent removal†
Abstract
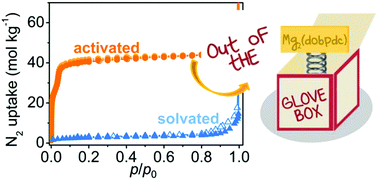
Mg2(dopbdc) (H4-dobpdc = 4,4′-dihydroxy-(1,1′-biphenyl)-3,3′-dicarboxylic acid) is an important MOF for its astonishing properties in gas separation and storage, in particular for applications related to CO2 separation. Its technological relevance is limited by its reputation for fast decomposition in air. In this article, this reputation is denied by showing that, if fully desolvated, the MOF is stable in air for 24 h (25 °C and 28–35 RH%), a time sufficiently long to allow its processing in a non-inert environment and its use as a CO2 scrubber in a wet post-combustion flow. In contrast, the material solvated with methanol showed a 93% decrease in surface area under the same conditions. Material activation is proposed here as a simple way to increase the stability of Mg2(dobpdc) in air, by the removal of preadsorbed polar molecules that can act as seeds for water condensation. This work allows us to make some important general considerations which can be taken into account in studies on material sensitivity to air. The importance of quantifing the aging time and testing the materials at different relative humidity is stressed. These parameters are important to determine, when possible, the conditions of use and storage, suitable for large scale processing and applications. Among the adopted characterization techniques (XRD, IR spectroscopy, N2 and CO2 volumetry), N2 volumetry at 77 K turned out to be the most suitable to evidence small variations in the structure due to decomposition. In contrast, CO2 uptake at RT and 1 bar was not a reliable quantity to evaluate MOF stability, because of the significant change in the (apparent) MOF affinity toward CO2 upon damaging.
- This article is part of the themed collection: 2017 Materials Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...