Investigation of supramolecular interactions between liquid crystals and PCBM for improved morphological stability in solar cells†
Abstract
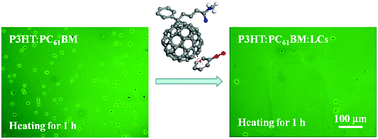
Supramolecular interactions between liquid crystals (LCs) of different chemical structures and PC61BM molecules have been studied, showing that 4-cyano-4′-pentylterphenyl (5CT) containing electron-withdrawing cyano substituents on the phenyl ring exhibited the strongest interactions with PC61BM, as revealed via density functional theory (DFT) calculations and experimental analysis based on Fourier transform infrared spectrometry (FTIR), differential scanning calorimetry (DSC) and polarized optical microscopy (POM). In contrast, 4-octyloxy-4′-cyanobiphenyl (8OCB) comprising an electro-donating octyloxyl group and dioctylterthiophene (8TTP8) with thiophene rings and long alkyl groups showed weaker interactions with PC61BM. After electric field treatment at 600 V mm−1 in an air environment, the P3HT:PC61BM:8OCB specimen showed a higher power conversion efficiency (PCE) of 2.9% and a more stable morphology than P3HT:PC61BM:8TTP8 with a PCE of 2.7%. Upon annealing at 150 °C for 1 h, 5CT is most effective in restricting the aggregation and crystallization of PC61BM molecules, thus stabilizing the morphology of P3HT:PC61BM. Moreover, the supramolecular interaction between LCs and PCBM could also influence the thermal stability in the narrow bandgap system of PTB7-Th:PC71BM, with 8TTP8 showing the highest ability to restrict the depression of the PCE value.


 Please wait while we load your content...
Please wait while we load your content...