Monolithic hierarchical gold sponges for efficient and stable catalysis in a continuous-flow microreactor†
Abstract
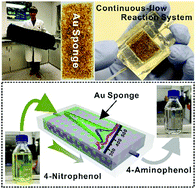
Monolithic hierarchical gold sponges are prepared by the polymer-assisted metal deposition method under air- and moisture-compatible conditions. These sponges feature excellent catalytic properties, robustness and chemical recyclability for the reduction reaction of 4-nitrophenol, and are also available for the scale-up catalysis of the same reaction in a continuous-flow microreactor.
- This article is part of the themed collections: Materials Chemistry Frontiers HOT articles for 2017 and MCF Editors’ Recommendation


 Please wait while we load your content...
Please wait while we load your content...