Synthesis and photophysical properties of a porphyrin–BODIPY dyad and a porphyrin–o-carborane–BODIPY triad†
Abstract
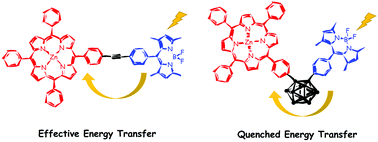
A novel porphyrin–BODIPY dyad, where BODIPY acts as the central antenna, linked via the Sonogashira coupling reaction, and a novel porphyrin–o-carborane–BODIPY triad, where both porphyrin and BODIPY are covalently attached to the o-carborane, have been synthesised and characterised. X-ray crystallography confirmed a V-shaped triad molecule. Detailed studies on the photophysical properties revealed the excitation of the BODIPY dyad system, which selectively triggered an efficient resonance energy transfer to the porphyrin unit. The presence of the o-carborane group in the triad system significantly diminished the energy transfer efficiency from BODIPY to the porphyrin moiety, because of its quenching properties. Besides, the triad system displayed aggregation-enhanced emission in THF/water systems, due to the presence of the o-carborane unit. The in-depth investigation of the electrochemical properties demonstrated that o-carborane insertion extended the initial reduction potentials and shifted half waves from the anodic to the cathodic site.


 Please wait while we load your content...
Please wait while we load your content...