Deep insight into the photocatalytic activity and electronic structure of amorphous earth-abundant MgAl2O4
Abstract
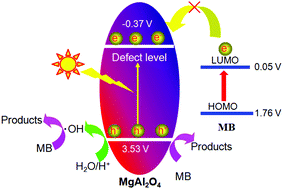
Developing inexpensive photocatalysts is of great value for industrial applications. In the work, magnesia–alumina spinel (MgAl2O4) made from earth-abundant elements is synthesized via a simple hydrothermal route and subsequent calcination. Theoretical calculation indicates that pure MgAl2O4 crystal has an energy band gap of 7.505 eV, and that the top of the valence band is mainly formed by hybridization between Mg-p and O-p states, while the bottom of the conduction band mainly originates from the Mg-s/p and O-s/p states. The UV-vis DRS results show that MgAl2O4 containing amorphous matter absorbs ultraviolet light with a band gap of 3.90 eV, which arises from the longer Mg–O bond length and presence of defects. This research also confirms that the visible-light photocatalytic activity reported in a previous paper is derived from the nitrogen element doping. The photocatalytic activity of MgAl2O4 is evaluated via the decomposition of methylene blue (MB) under UV-vis light illumination. The electron transfer route and the photocatalytic mechanism of MgAl2O4 for degrading MB are proposed. For the first time, the potentials of the energy bands of MgAl2O4 are obtained using Mott-Schottky electrochemical measurement.


 Please wait while we load your content...
Please wait while we load your content...