Intermediate bands of MoS2 enabled by Co doping for enhanced hydrogen evolution†
Abstract
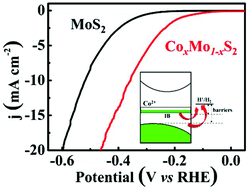
MoS2 is a promising non-noble metal electrocatalyst for hydrogen evolution reaction (HER). Transition metal doping (TM = Fe, Co, Ni, etc.) into the Mo site of MoS2 is a simple and effective method to improve the catalytic activity of MoS2. However, homogeneous TM-doping suffers from phase segregation due to the different coordination modes between TMS6 and MoS6. Here, we propose a solid-state reaction method with rapid heating and quenching to homogeneously dope TM atoms into the MoS2 lattice. The electrical conductivity of a Co-doped sample (CoxMo1−xS2) is ten times larger than that of pristine MoS2. The CoxMo1−xS2 is applied as an efficient electrocatalyst for the hydrogen evolution reaction, which has an onset potential of HER activity near −65 mV versus RHE and a Tafel slope of 120 mV dec−1, compared with pristine MoS2 (an onset potential of −240 mV versus. RHE, a Tafel slope of 133 mV dec−1). The first-principles calculations reveal that half-filled intermediate bands (IBs) mainly consist of Co 3d orbitals present in the forbidden band of 2H-MoS2 after Co doping. Half-filled IBs in CoxMo1−xS2 account for better electrical conductivity and lower overpotential to promote rapid electron transfer to hydrogen ions, consequently resulting in efficient hydrogen evolution.


 Please wait while we load your content...
Please wait while we load your content...