One-step synthesis of CoO/g-C3N4 composites by thermal decomposition for overall water splitting without sacrificial reagents†
Abstract
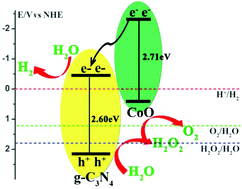
Using photocatalysts to split water into hydrogen and oxygen is a promising way to produce renewable energy. Efficient and stable photocatalysts are desired for overall water splitting under visible light. We demonstrate that the cobalt(II) oxide (CoO)/graphitic carbon nitride (CoO/g-C3N4) composites synthesized by a one-step thermal decomposition method exhibit enhanced photocatalytic efficiency for overall water splitting without sacrificial reagents. 10%CoO/g-C3N4 exhibits optimal photocatalytic performance, where H2 and O2 evolution rates are, respectively, 0.46 μmol h−1 and 0.21 μmol h−1 under visible light irradiation without any sacrificial reagents. It is worth mentioning that the H2 and O2 production almost approaches the stoichiometric ratio 2 : 1. The enhanced photocatalytic activity of CoO/g-C3N4 may be ascribed to the efficient charge separation and fast decomposition of H2O2 by CoO.


 Please wait while we load your content...
Please wait while we load your content...